A Gut Feeling About Alzheimer’s
So, Alzheimer’s disease, huh? It’s this relentless, heartbreaking thing, isn’t it? The most common form of dementia slowly, inexorably steals memories, personalities… everything. And for all our brilliant minds, for all the years of tireless research, truly effective treatments? Well, they’ve been elusive, to say the least. It’s why we’re always, always on the hunt for fresh ideas, new angles, anything that might crack this incredibly tough nut.
Now, here’s the kicker: what if it all starts… in your gut? Seriously. This isn’t just some wild theory; it’s a groundbreaking new hypothesis, one that’s really shaking things up. It points to a specific pathway, involving a rather potent neurotoxin, BF-LPS, which is actually churned out by a super common gut bacterium, Bacteroides fragilis. This report, you see, is going to dive deep into the science, the nitty-gritty mechanisms, showing how these gut-derived factors, yes, from your very own digestive system, might be playing a huge, perhaps even initiating, role in Alzheimer’s. We’re talking about their impact on brain cells, inflammation, and even those notorious protein clumps we associate with AD.
Beyond that, we’ll explore what this means for understanding the disease, for finding new ways to spot it early (biomarkers, they call them), and, crucially, for developing interventions that target the gut itself. Imagine: diagnosing, preventing, even treating Alzheimer’s by focusing on gut health. It’s a pretty compelling vision, if I’m honest. This emerging understanding of the gut-brain axis, this incredible, two-way street between your belly and your brain, well, it offers a truly promising frontier. It could totally transform how we approach Alzheimer’s, making lifelong gut health not just a good idea, but a critical piece of the puzzle for keeping our brains sharp and healthy.
The Gut, the Brain, and a Lifelong Mystery
Alzheimer’s disease, or AD as it’s often called, is, without a doubt, the leading cause of dementia. It’s a cruel thief, relentlessly taking away cognitive function, memory, and even the ability to regulate one’s own behavior. When we look at the brains of people with AD, what do we see? Well, there are these tell-tale signs: sticky clumps of amyloid-beta, or Aβ, outside the cells, and twisted tangles of hyperphosphorylated tau protein, called NFTs, inside the neurons. These aren’t just innocent bystanders; they’re known to kick off inflammation in the brain, cause synapses, those vital communication points between neurons, to wither, and ultimately, lead to widespread brain cell death.
For decades, scientists have been poring over this, trying to unravel the incredibly complex, multifaceted reasons why AD happens. And despite all that effort, finding truly effective treatments to stop or even slow its march? It’s been incredibly tough, a real uphill battle. That’s why we desperately need fresh perspectives, new ways of looking at what’s really going on.
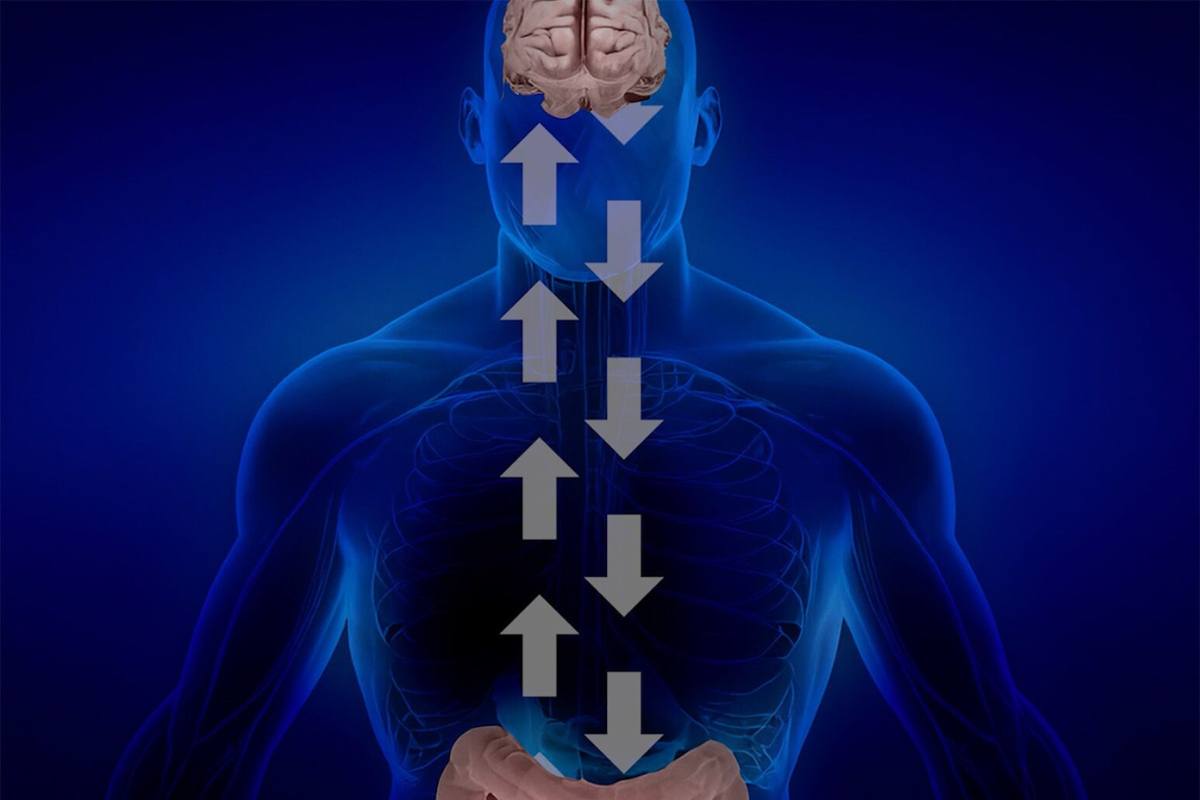
Enter the gut-brain axis, or GBA. This concept has really exploded in recent years, describing this amazing, continuous, two-way conversation that happens between your central nervous system, your brain and spinal cord, and your gastrointestinal tract. And believe it or not, there’s this rapidly growing appreciation for just how much influence your gut microbiota, those trillions of tiny organisms living inside you, have on your neurological health, for better or worse. This intricate communication system, sometimes called the “brain-gut-microbiota axis” (a mouthful, I know), works through all sorts of channels: nerves, immune cells, hormones, and even metabolic signals. It’s quite the network.
Now, here’s where it gets really interesting, and frankly, a bit mind-blowing. A recent, truly groundbreaking hypothesis, the kind that makes headlines, suggests that Alzheimer’s disease might actually start in the gut. This isn’t just a minor tweak to our understanding; it’s a radical shift. It’s based on cutting-edge research that points to a new, direct pathway for AD to begin, with a potent toxin, produced by a common gut bacterium, acting as the initial spark.
This idea, that AD might not be solely a brain problem but one with origins in our digestive system, well, it forces us to take a much broader, more holistic view of the disease. It’s not just about finding another risk factor; it’s about identifying a potential starting point for the whole devastating cascade.
And the implications? Oh, they’re huge. If this AD-linked pathway truly begins inside the body and stays active throughout our lives, it completely changes when and how we can intervene. If the disease process is a chronic, lifelong affair, deeply influenced by gut health, then prevention isn’t just about treating symptoms late in the game. It means we could potentially target gut health much, much earlier, maybe even decades before anyone shows a single sign of cognitive decline. This opens the door to true primary prevention, moving beyond just managing symptoms to actually stopping the disease from taking hold. It also broadens our idea of “healthy aging” to explicitly include proactive gut health management. It’s a whole new ballgame for understanding AD and developing innovative ways to diagnose and treat it.
The Gut-Brain Axis: The Foundation of a Healthy Mind
Your human gastrointestinal tract is just incredible, isn’t it? It’s home to this unbelievably dense and diverse ecosystem of microorganisms, which we call the gut microbiome. We’re talking trillions of bacteria, fungi, parasites, and even viruses. And this vast microbial community? It’s not just hanging out; it’s actively involved in all sorts of essential bodily functions. Think about it: developing your immune system, digesting your food, and, perhaps most surprisingly, profoundly influencing the overall health of your brain. The gut-brain axis, or GBA, is the grand framework for this complex, two-way conversation between these gut microbes and your central nervous system.
This communication along the GBA it’s a marvel of complexity, involving a sophisticated dance of various signaling pathways. Neural signaling, for instance, is a big one. Your enteric nervous system, often affectionately dubbed your “second brain,” has this remarkable autonomy in controlling how your gut works. And it’s intimately connected to, and influenced by, your main brain through sympathetic and parasympathetic nerves, with the vagus nerve acting as this crucial, direct highway for communication.
But it’s not just direct nerve lines. Immune signaling plays a huge part, too. The gut microbiota, those tiny residents, significantly shapes how your immune cells behave and what kind of inflammatory signals, or cytokines, they produce. These systemic immune responses, in turn, can directly affect inflammation, both throughout your body and right there in your brain, forming a critical link within the GBA.
Then there’s endocrine signaling. Your gut microbes, believe it or not, can churn out a whole host of neuroactive compounds and hormones. These substances can then hop into your bloodstream, travel around, and ultimately influence how your brain functions and even your behavior.
And finally, metabolic pathways are absolutely key to GBA communication. We’re talking about microbial metabolites here. Short-chain fatty acids, or SCFAs, for example, are produced by gut bacteria when they ferment dietary fibers. These are vital, not just for a happy gut, but for brain function too. They can actually cross the blood-brain barrier, that protective shield around your brain, and tweak the activity of microglial cells, which are basically the brain’s resident immune cells, thereby regulating inflammatory responses right there in the central nervous system. Similarly, tryptophan derivatives, which are products of microbial metabolism, also play roles in brain function; some are protective, others, well, not so much.
Now, when this delicate balance in the gut microbiome gets thrown off, we call it gut dysbiosis. And it’s increasingly recognized as a major player in GBA impairment and, sadly, in the development of neurodegenerative diseases. This dysbiotic state can lead to something often called “leaky gut,” where the gut barrier, that protective lining, becomes more permeable. What happens then? Bacteria-derived products and pro-inflammatory cytokines can sneak into the bloodstream. This systemic inflammation, a real troublemaker, can then compromise the integrity and function of the blood-brain barrier, promote inflammation within the brain, cause neural injury, and, ultimately, contribute to neurodegeneration.
And here’s the thing: in people with Alzheimer’s disease, we often see these changes in the gut microbiome. It’s usually a decrease in microbial diversity and stability, and a shift towards a more pro-inflammatory bacterial profile. This detailed understanding of GBA communication reveals something rather unsettling: gut dysbiosis doesn’t just contribute to AD; it sets up a self-reinforcing, bidirectional amplification loop. The initial gut barrier dysfunction leads to systemic inflammation, which then weakens the blood-brain barrier, allowing even more gut-derived toxins to flood into the brain and further fan the flames of neuroinflammation. And a compromised blood-brain barrier, in turn, allows even more harmful substances to cross into the brain, exacerbating the neuroinflammatory response and accelerating the whole pathological cascade. It’s a vicious cycle, you see.
These intricate connections within the GBA suggest that keeping your gut microbiome healthy isn’t just a specific intervention for AD; it’s a fundamental strategy for building overall brain resilience against all sorts of neurodegenerative insults. The GBA’s influence, after all, extends to broad “human neurological health and disease.” The way gut dysbiosis leads to blood-brain barrier impairment implies that a compromised gut barrier weakens the brain’s defenses against any harmful substances, not just those specific to AD. So, a healthy, diverse, and stable gut microbiome, by maintaining the integrity of both your gut and your blood-brain barrier, provides a foundational layer of protection. This elevates gut health beyond just a targeted AD therapy to a general principle for promoting neurological well-being and reducing susceptibility to various forms of neurodegeneration. It’s almost like saying gut health is a prerequisite for optimal brain function, for a lifetime.
Specific Mechanisms: How Gut Factors Might Be Driving Alzheimer’s Pathology
The gut microbiome it’s a busy place, and it seems it contributes to Alzheimer’s disease pathology through a really complex dance of multiple, interconnected pathways. These mechanisms they involve everything from direct neurotoxin delivery to generalized inflammatory responses, even inducing protein misfolding, and messing with your metabolism. All of these, mind you, converge to exacerbate the core pathologies we see in AD.
The BF-LPS Pathway: A Direct Line from Your Gut to Your Brain
Now, let’s talk about some cutting-edge research, the kind that makes you sit up and take notice. Drs. Yuhai Zhao and Walter J. Lukiw, over at the LSU Health New Orleans Neuroscience Center, they’ve really been at the forefront, identifying Bacteroides fragilis as a crucial bacterium in the whole AD story. This common Gram-negative bacterium, a natural resident of your gastrointestinal tract, produces a potent neurotoxin. It’s called BF-LPS, a lipopolysaccharide. And Dr. Lukiw, he doesn’t mince words; he says lipopolysaccharides, including this BF-LPS, are considered “among the most potent microbial-derived pro-inflammatory neurotoxic glycolipids known.” Pretty serious stuff, right?
The research meticulously traces the journey of this BF-LPS. Under certain conditions, and this is key, this toxin can actually breach the gut lining, slip into your bloodstream, and then, believe it or not, cross that protective blood-brain barrier to infiltrate various parts of your brain. The permeability of both the gut and blood-brain barriers, it turns out, can notably increase as we age and as diseases progress, making it easier for these harmful microbial products to sneak through.
Once it gets into the brain, BF-LPS directly triggers inflammation right there in the neurons. And it’s not just a theory; it’s well-established that various forms of LPS have been found within neurons affected by Alzheimer’s disease in multiple labs. BF-LPS, specifically, has been pinpointed as an exceptionally potent inducer of a pro-inflammatory transcription factor called NF-κB (the p50/p65 complex, if you want to get technical). This NF-κB, by the way, is a known trigger in the expression of pathogenic pathways involved in inflammatory neurodegeneration.
But here’s a critical finding, a real “aha!” moment: BF-LPS directly inhibits the production of neurofilament light chain, or NF-L. What’s NF-L, you ask? Well, it’s a vital protein, a core component of the neuronal cytoskeleton, acting like a crucial scaffolding element for the axoskeleton. It’s absolutely essential for keeping nerve cells structurally stable and intact, supporting their shape, their internal architecture, how they form connections (synaptogenesis), and how they transmit signals (neurotransmission). Without it, things start to fall apart.
The study went even further, showing that BF-LPS activates the NF-κB signaling pathway within brain cells. And this activation of NF-κB, in turn, significantly boosts the production of a tiny RNA molecule, microRNA-30b (miRNA-30b). This microRNA-30b then directly latches onto NF-L messenger RNA, leading to an even further reduction in NF-L protein production. It’s like a “double blow” mechanism, where the BF-LPS toxin not only directly messes with NF-L but also activates a genetic suppressor, making the deficiency even worse.
And get this: overexpression of microRNA-30b in the hippocampus, that part of your brain crucial for memory, has been shown to impair basic synaptic transmission, reduce those little tree-like branches on neurons called dendritic spines, and negatively affect learning and memory. Sound familiar? It closely mimics the cognitive deficits we see in AD. This discovery it’s a first: the first identified instance of a microbiome-derived neurotoxin altering neuron structure through microRNA-mediated gene suppression. The result of all this? That drop in intracellular NF-L levels due to BF-LPS exposure leads to the collapse of the axoskeleton, causing neurons to shrink, axons to thin, synapses to get disrupted, and ultimately, cell death. These are widespread, characteristic pathological changes, mind you, observed in the brains of Alzheimer’s patients. And this structural degradation? It directly compromises neurotransmission, memory formation, and overall brain function. It’s a big deal.
The continuous production of neurotoxins like BF-LPS, as natural byproducts of gut microbial metabolism, combined with the age-related increases in gut and blood-brain barrier permeability, well it paints a picture. It suggests a model where AD could be seen as the cumulative result of chronic, low-grade systemic insults, originating right there in the gut, throughout a person’s entire life. The research it explicitly states that BF-LPS is a “natural byproduct of GI-tract-based microbial metabolism” and that this AD-linked pathway “begins inside the body… and is active throughout life.” At the same time, scientific literature points out that gut and blood-brain barrier permeability “alter or increase with aging and disease.”
So, when you put all that together, it leads to this understanding: your brain is potentially under a continuous, albeit low-level, assault from gut-derived toxins over many, many decades. This shifts how we think about AD, from a disease with a sudden, late-life onset to a slow, cumulative process of “wear and tear” on the brain, made worse by the natural physiological changes that come with aging. This model has profound implications, really emphasizing that lifelong gut health management is a critical piece of preventative care for AD. It’s something to chew on, for sure.
Broader Gut Microbiota Contributions to AD Pathogenesis
Beyond the specific effects of BF-LPS, there’s a whole host of ways the general gut microbiota contributes to AD. In older folks, age-related shifts in the gut microbiome, less diversity, less stability, can lead to this persistent, low-grade inflammation in the gut lining, a phenomenon we call “inflammaging.” This dysregulation causes a breakdown of the intestinal barrier, the “leaky gut” we talked about. And what happens then? More pro-inflammatory cytokines and bacteria-derived products can sneak into the bloodstream. This systemic inflammation, in turn, compromises the integrity of the blood-brain barrier and fuels neuroinflammation right there in the brain. What’s more, specific pathogenic bacterial products, like exotoxins from E. coli, Salmonella, and yes, Bacteroides fragilis, can directly mess with the integrity of those epithelial cells and their tight junctions in the gut lining.
The gut microbiota it’s a significant source of amyloids, too. Curli, produced by Escherichia coli, is one of the most studied bacterial amyloids. And here’s the fascinating part: even though their primary amino acid sequences are different, these bacterial amyloids share striking similarities in their three-dimensional β-sheet structures with the amyloids we find in the brain, like Aβ and tau. Exposure to bacterial amyloid proteins in the gut can actually “prime” your immune system, leading to an exaggerated immune response when your brain starts producing its own neuronal amyloid. This process, dubbed “mapranosis” (microbiota-associated proteopathy and neuroinflammation), suggests that bacterial amyloids can act like prion-like proteins through something called molecular mimicry. In this scenario, a bacterial amyloidogenic protein (like curli) can trick a host protein (like Aβ or tau) into adopting a pathogenic β-sheet shape, thereby speeding up its aggregation and misfolding. It’s a bit like a bad influence, if you will.
And it’s not just bacterial amyloids. General bacterial lipopolysaccharides, or LPS, have been shown to reproduce key inflammatory and pathological features of AD when injected into the brains of animal models. LPS can promote amyloid fibril formation and induce a more pathogenic β-pleated sheet conformation of prion amyloids in lab studies. LPS activates Toll-like receptors, or TLRs, especially TLR4 and TLR2, which are found on microglial cells. Remember, those are the brain’s resident immune cells, thereby triggering strong inflammatory responses. Interestingly, these very same TLRs are also triggered by Aβ and bacterial amyloids, further supporting the idea of molecular mimicry as a link between gut and brain pathology.
Your gut microbes also produce a whole diverse range of metabolites that significantly influence brain function. Short-chain fatty acids, or SCFAs, generated by gut bacteria from the fermentation of dietary fibers, are crucial for both digestive health and brain function. SCFAs, particularly butyrate, can impact brain health by modulating the integrity of the blood-brain barrier, reducing neuroinflammation, and even improving the growth of new brain cells, neurogenesis. But, and this is important, too much of a good thing can be, well, not so good; excessive levels of SCFAs might potentially interfere with microglial activity or even induce amyloid β formation. Tryptophan derivatives, part of the kynurenine pathway of tryptophan metabolism, are also involved in brain function and mood regulation. While some derivatives, like kynurenic acid, offer neuroprotection, others, like quinolinic acid, are neurotoxic and can cause inflammation and cell death. Plus, the metabolism of bile acids by gut microbes is a key physiological process, and impaired gut bacteria-metabolized bile acids have been observed in AD, hinting at their role in gut-to-brain communication.
An altered threshold for microglial activation, which we see in neurodegeneration and aging, might stem from repeated or chronic systemic infections or inflammatory exposures. Microglial cells that have been “primed” by exposure to bacterial amyloids or LPS may become hyper-responsive to subsequent stimuli, like Aβ in the brain, leading to an exaggerated and harmful inflammatory response that speeds up neurodegeneration. It’s a snowball effect.
So, what does all this tell us? The research reveals that the gut microbiome doesn’t just contribute to AD through one isolated mechanism. Oh no, it’s a complex interplay of multiple, interconnected pathways. These pathways, direct neurotoxin delivery, generalized inflammatory responses, inducing protein misfolding, and metabolic dysregulation, all converge and exacerbate the core pathologies of AD, things as inflammation, amyloid and tau aggregation, and neuronal damage. This isn’t just a collection of independent events; it’s a complex, self-reinforcing system. Gut dysbiosis acts as a central hub, initiating and worsening AD pathology through multiple, synergistic mechanisms. This implies that effective therapeutic strategies might need to take a multi-target approach, focusing on overall gut health, rather than just narrowly targeting a single pathway. It’s a big picture kind of problem, you see.
Key Gut-Derived Factors and Their Mechanisms in Alzheimer’s Disease Pathogenesis
| Factor | Source/Origin | Pathway to Brain | Key Mechanism(s) in AD | Impact on AD Pathology |
| BF-LPS | Bacteroides fragilis (Gram-negative bacterium) | Leaks from the GI tract cross the BBB via circulation | NF-κB activation, microRNA-30b upregulation, NF-L inhibition | Neuronal atrophy/death, synaptic loss, cognitive decline, structural damage |
| General Lipopolysaccharides (LPS) | Gram-negative bacteria | Leakage from the GI tract crosses the BBB, systemic inflammation | Neuroinflammation, amyloid fibrillogenesis, TLR activation, microglial priming | Exacerbates Aβ/tau aggregation, neuronal damage, and cognitive deficits |
| Bacterial Amyloids (e.g., Curli) | Escherichia coli (gut microbiota) | Leakage from the GI tract, systemic immune priming | Molecular mimicry, cross-seeding of Aβ/tau, immune system priming (“mapranosis”) | Promotes Aβ/tau misfolding and aggregation, neuroinflammation |
| Short-Chain Fatty Acids (SCFAs) | Gut microbiota fermentation of dietary fiber | Crosses BBB, systemic circulation | BBB modulation, neuroinflammation reduction, neurogenesis improvement; potential Aβ induction (excessive) | Modulates brain health; potential for both beneficial and detrimental effects |
| Tryptophan Derivatives | Gut microbiota metabolism | Systemic circulation crosses the BBB | Neurotoxicity (e.g., quinolinic acid) or neuroprotection (e.g., kynurenic acid) | Contributes to inflammation and cell death or brain protection |
Export to Sheets
Implications for Alzheimer’s Disease: Etiology, Biomarkers, and Prevention
Integrating the Gut-Centric Hypothesis with Existing AD Models
So, for a long time, the main ideas about Alzheimer’s disease pathogenesis have been the amyloid cascade hypothesis, which basically says Aβ aggregation is the first domino to fall, leading to tau pathology and then neuronal death, and the tau hypothesis, suggesting tau phosphorylation and aggregation might be the primary cause, maybe even before Aβ shows up. These ideas they’ve really dominated research, no doubt about it. But, and this is a big “but,” the consistent failures of clinical trials that specifically targeted Aβ have really made us all step back and take a hard look.
Now, the emerging gut-centric hypothesis, this new kid on the block, it doesn’t necessarily throw out those established models. Not at all. Instead, it offers a crucial upstream or parallel initiating factor. Think of it this way: gut-derived factors, like lipopolysaccharides (LPS) and bacterial amyloids, they’ve been shown to directly promote amyloid fibril formation and even induce those pathogenic β-sheet shapes in both Aβ and tau proteins. How? Through mechanisms like molecular mimicry and immune priming. This integration it suggests that gut dysbiosis and its associated products could be significant triggers or accelerators for both amyloid and tau pathologies, thereby weaving the gut right into the broader, multifactorial tapestry of AD etiology. It’s a more complete picture, you see.
The new research it explicitly highlights that this AD-linked pathological pathway can actually originate in the gut and, get this, remains active throughout an individual’s entire life. That’s a profound implication, isn’t it? It suggests that neurotoxins and inflammatory mediators coming from the gut aren’t just secondary consequences of AD; they have the potential to be primary initiators or, at the very least, significant exacerbators of AD pathology. And when you combine chronic, gut-derived inflammatory responses with the natural processes of aging and less-than-ideal dietary patterns in older adults, well, that’s recognized as a substantial contributor to AD pathogenesis. It’s a perfect storm, in a way.
Emerging Biomarkers from the Gut-Brain Axis
Neurofilament light chain, or NF-L, is a protein specific to neurons. When there’s axonal damage or degeneration, it gets released in pretty significant quantities into the cerebrospinal fluid, and then, you guessed it, into the blood. Now, while intracellular NF-L levels actually decrease within neurons during degeneration, notably because of the BF-LPS-induced overexpression of microRNA-30b, its elevated levels in blood and CSF make it a really promising biomarker. NF-L can be used for diagnosing and tracking the progression of various neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and ALS. But, and this is important to remember, NF-L isn’t specific to AD. Its levels go up no matter what kind of neuropathological damage is causing axonal loss. Plus, blood NF-L levels actually correlate positively with age, so you need to use age-dependent reference values for accurate interpretation. It’s a good tool, but you have to know how to use it.
MicroRNA-30b, or miRNA-30b, is another interesting one. It’s found to be strongly upregulated in Alzheimer’s brains, especially when neurons are exposed to LPS or amyloid-beta. And its overexpression in the hippocampus, as we discussed, has been shown to impair synaptic transmission, reduce dendritic spines, and negatively affect learning and memory. Given that it directly targets the NF-L gene, which, remember, messes with the neuron’s structural backbone, blocking the effects of microRNA-30b could potentially help preserve NF-L and keep synapses stable. This makes microRNA-30b a compelling candidate, not just for a specific biomarker, but also for a novel therapeutic target in AD. It’s a small molecule, but with potentially big impact.
Changes in the composition of the gut microbiota and the profiles of their metabolites, like short-chain fatty acids (SCFAs), are actively being investigated as potential biomarkers for the early detection and monitoring of AD progression. Specific microbial populations and their metabolic byproducts are increasingly being linked to AD pathology. And advanced multi-omics approaches, integrating data from the gut microbiome, metabolomics, and neuroimaging, are being used to build more robust diagnostic models, especially for those preclinical AD stages, before symptoms even show.
The convergence of these gut-derived pathogenic mechanisms, gut-influenced biomarkers (NF-L, microRNA-30b, microbial signatures), and gut-targeted interventions (like dietary fiber, specific diets) really positions the gut-brain axis as a cohesive and accessible platform for both AD diagnosis and therapy. The discovery of the BF-LPS pathway it provides a direct mechanistic link between the gut and AD pathology. At the same time, NF-L and microRNA-30b are identified as potential biomarkers whose levels are influenced by this gut-derived pathology. And then, dietary fiber is proposed as a simple, accessible intervention to modulate the very source of the neurotoxin. This creates a powerful, integrated framework: a pathology originating in the gut, measurable biomarkers influenced by it, and modifiable gut-based interventions. It implies that future AD management could involve a unified approach where diagnosis (through accessible gut-related biomarkers) and prevention or treatment (through gut modulation) are intrinsically linked, offering a less invasive and potentially more practical strategy compared to current brain-centric methods. It’s a pretty exciting prospect, if you ask me.
Dietary and Lifestyle Prevention Strategies
Dietary fiber it plays a crucial role, doesn’t it? It helps regulate the composition of your gut microbiome. By simply eating more fiber, you can promote a balanced microbial community, which, in turn, can limit the abundance of Bacteroides fragilis. And what does that mean? Less of its neurotoxin, BF-LPS, is produced, thereby lowering the risk of this toxin ever reaching your brain. Dr. Lukiw really emphasizes this: balancing the microorganisms in your microbiome through dietary approaches can effectively regulate those AD-related microbes and their potential to release neurotoxins like BF-LPS. It’s a simple, yet powerful, idea.
The type of diet you eat it’s a paramount factor, truly, in shaping your gut microbiota. Healthy dietary patterns, especially those plant-based diets rich in fiber, polyphenols, and unsaturated fats, they’re known to foster beneficial bacteria in the gut. Such diets are consistently linked to a healthier gut microbiome and, quite demonstrably, a lower risk of cognitive decline. On the flip side, diets packed with high sugar, high cholesterol, and high-fat content, well, they’ve been shown to negatively impact gut microbiota composition and, sadly, adversely affect cognitive abilities, including your spatial memory. Food for thought, literally.
Beyond what you eat, other environment-based lifestyle factors, like getting regular physical exercise and managing stress effectively, are increasingly recognized for their ability to influence and modulate the gut microbiome. This hints at their potential as novel and accessible preventive strategies for AD. The inherent variability in each person’s gut microbiome composition and how they respond to dietary interventions, though, it really underscores the critical need for personalized approaches in AD prevention and management. We’re talking about moving beyond generic dietary advice here. Scientific literature it keeps emphasizing that diet modifies the gut microbiota and that “individual variations in microbiome composition necessitate individualized therapeutic approaches.” The fact that the abundance of specific bacteria like Bacteroides fragilis can be regulated by dietary fiber implies that the effectiveness of such interventions will likely vary significantly between individuals, depending on their unique baseline microbiome and dietary habits. This leads to a broader understanding: a universal “one-size-fits-all” dietary or probiotic recommendation for AD prevention might just not be enough. Instead, a future of precision nutrition and personalized microbiome modulation, tailored to your specific gut profile, is suggested as a more effective strategy for reducing AD risk and managing the disease. It’s a fascinating thought, isn’t it?
Therapeutic Approaches: Targeting the Gut Microbiome for AD
The gut microbiome is not just one therapeutic target for AD, you see. Oh no, it’s more like a diverse and ever-expanding arsenal of multi-modal interventions. We’re talking everything from broad dietary tweaks to highly specific microbial or molecular modulations. It’s a whole spectrum of possibilities.
Probiotics and Prebiotics: Your Gut’s Best Friends
Probiotics are live microorganisms that, when you take enough of them, actually give you a health benefit. They work their magic on the microbiota-gut-brain axis mainly by tweaking your gut microbiota, producing healthy fermentation products, and just directly interacting with your body. Some of their specific tricks include making your intestinal barrier stronger by boosting certain proteins that hold cells together, reducing oxidative stress, and lowering those pesky pro-inflammatory cytokines.
Prebiotics, on the other hand, are non-digestible food ingredients. They’re like special food for your gut bacteria, selectively metabolized by them, leading to specific, beneficial changes in the composition and activity of your gut microbiome. When your gut bacteria ferment them, they churn out beneficial short-chain fatty acids (SCFAs) and lactic acid. It’s a win-win, really.
And the evidence? Well, it’s pretty compelling, from both preclinical studies and clinical trials. We’ve seen that taking probiotics can actually have a positive impact on anxiety, cognitive status, and even how well older adults with mild to moderate Alzheimer’s can handle their daily tasks. Plus, studies in middle-aged rats showed that symbiotic supplements, that’s a combo of probiotics and prebiotics, significantly improved spatial memory. And this improvement came with some really good stuff: lower inflammation, more brain-derived neurotrophic factor (BDNF, which is great for memory), and higher levels of butyrate, all known to boost memory formation and improve brain cell activity.
Specific probiotic strains, like Lactobacillus plantarum MTCC1325, have even been shown to help with cognition problems and significantly increase acetylcholine levels in animal models of AD. And get this: in a Parkinson’s disease mouse model, daily probiotic consumption for 16 weeks actually showed a neuroprotective impact and lessened the progressive impairment of motor activity. Immunohistochemical staining even revealed more intact dopamine neurons in the probiotic-treated group. Pretty amazing, right? Probiotic treatment in mice has also been shown to reverse the negative effects of antibiotics on gut bacteria (dysbiosis) and improve memory function. And in a randomized, double-blind, placebo-controlled clinical trial with Parkinson’s patients, taking probiotics and vitamin D together actually reduced inflammatory cytokines and increased anti-inflammatory ones. Prebiotics, too, have been shown to reduce pro-inflammatory bacteria like Proteobacteria and Escherichia coli while boosting those good SCFA-producing species. One exploratory study with AD patients even found that probiotic consumption led to a drop in zonulin concentrations, a known marker of intestinal barrier integrity. It’s all pretty encouraging, if you ask me.
5.2. Novel and Emerging Interventions: Beyond the Basics
Researchers at the Indiana University School of Medicine are really digging into the intricate connection between niacin homeostasis and the gut microbiome in the context of AD. Their big idea? It centers on the thought that specific bacteria living in your gut actually produce niacin, a B vitamin that’s super important for brain health, and for regulating blood sugar, metabolism, and cholesterol levels. In AD, they hypothesize, the pathway responsible for this niacin production in the gut might get all messed up, leading to a niacin deficiency. A key part of their hypothesis is that a lower supply of niacin to the brain could reduce the activation of the niacin receptor (HCAR2) in those brain-resident immune cells, the microglia, potentially driving AD progression. So, modulating niacin-producing bacteria or even pharmacologically activating this pathway is being explored as an easily accessible therapeutic target. A clinical trial for niacin in AD actually kicked off in 2024. Talk about moving fast!
Fecal Microbiota Transplantation, or FMT, is exactly what it sounds like. It involves taking fecal material from a healthy donor and putting it into the gastrointestinal tract of a recipient. The main goal? To restore an imbalanced gut microbiota to a healthier, more beneficial composition. Preclinical studies have shown some really promising results. FMT from wild-type mice into AD animal models, like those with certain transgenes, actually helped reduce the formation of Aβ plaques and neurofibrillary tangles, lessened glial reactivity, and even improved cognitive impairment. What’s more, clinical case reports have hinted at rapid improvement in AD symptoms after just a single FMT infusion. While it’s promising, we definitely need more robust clinical evidence. And FMT, it’s currently being explored for its potential in other neurodegenerative disorders too.
Now, when it comes to microbiome research, antibiotics are mostly used as a tool to broadly and non-specifically manipulate microbial communities for experimental purposes. But here’s the thing: their non-specific eradication of both pathogenic and beneficial bacteria can lead to significant dysbiosis and other unwanted side effects, making them potentially less than ideal for direct clinical use in AD patients. Research is still ongoing, though, to see if antibiotic use after AD symptoms appear can effectively alleviate pathology and symptoms. It’s a tricky balance.
And postbiotics? These are defined as beneficial substances produced by bacteria during their growth, like those short-chain fatty acids we talked about. While they hold promise, the research on their specific effects on AD pathologies is still in its very early stages. It’s just too soon to recommend or consider postbiotics as established therapeutic agents for AD. We need a lot more extensive research, that’s for sure. Future advancements in this field might involve some really high-tech biotechnological approaches, including microbial encapsulation, using bacteriophages therapeutically, employing microbial enzyme modulators, and even engineering microbes to produce specific beneficial metabolites. All of this, of course, to allow for more controlled and efficient interventions. It’s a brave new world, really.
Many of these gut-targeted interventions, especially the dietary and probiotic ones, inherently serve a dual role. They effectively blur that traditional line between disease prevention and active treatment, suggesting a continuum of care across the entire AD spectrum. Dietary fiber, for instance, is presented as a preventative measure by regulating Bacteroides fragilis abundance. At the same time, probiotics and prebiotics are discussed for both improving cognition in existing AD patients and potentially delaying pathology. This really shows that the same principles of maintaining a healthy gut that might prevent AD onset can also be used to manage or slow its progression once symptoms have appeared. It suggests a shift towards a continuous, lifelong management strategy where lifestyle and microbiome modulation are central components, moving beyond that rigid divide of “prevention” before diagnosis and “treatment” after. This has significant implications for how healthcare providers might advise patients at various stages of AD risk or progression. It’s a holistic view, and frankly, it makes a lot of sense.
Microbiome-Targeted Interventions for Alzheimer’s Disease: Mechanisms and Evidence
| Intervention Type | Proposed Mechanism(s) of Action | Key Evidence/Findings | Current Status/Limitations |
| Dietary Fiber | Regulates B. fragilis abundance; reduces BF-LPS production; balances gut microbiome | Reduces BF-LPS production, lowers the risk of toxin reaching the brain | Promising dietary approach; foundational for gut health |
| Probiotics | Modulates gut microbiota; enhances intestinal barrier; reduces oxidative stress/inflammation; improves cognition | Improved cognition, reduced anxiety, enhanced daily functions in AD patients; neuroprotection in animal models | Promising, but more robust human clinical trials are needed |
| Prebiotics | Promotes beneficial bacteria growth; induces SCFA production; modulates gut microbiota composition | Improved spatial memory, increased BDNF, reduced pro-inflammatory bacteria, and improved intestinal barrier integrity | Promising, but more robust human clinical trials are needed |
| Niacin Modulation | Influences HCAR2 receptor in microglia via gut bacteria; regulates niacin levels for brain health | Reduced plaques, improved cognition in animal models; clinical trial initiated in 2024 | Early clinical trial stage; potential as an accessible therapeutic target |
| Fecal Microbiota Transplantation (FMT) | Restores imbalanced gut microbiota to a more beneficial composition | Ameliorated Aβ plaques/NFTs, reduced glial reactivity, improved cognition in animal models; case reports of symptom improvement | Promising, but requires more rigorous clinical evidence and standardization |
| Antibiotics | Broadly manipulates microbial community; targets harmful bacteria | Used as an experimental tool; some epidemiological data suggest decreased dementia likelihood | Not ideal for direct clinical AD application due to dysbiosis risk; non-specific; more research on post-symptomatic use needed |
| Postbiotics | Beneficial substances produced by bacteria (e.g., SCFAs) | Specific effects on AD pathologies are under investigation | Too early for recommendation; requires extensive further research |
Export to Sheets
Current Research Landscape, Challenges, and Future Directions
The scientific community it’s really buzzing, actively engaged in investigating those intricate bacterial connections between the gut microbiome and the progression of Alzheimer’s disease. Right now, several clinical trials are actually underway, exploring what happens when AD patients take probiotic supplements. And the preliminary observations? They’re hinting at possible benefits in cognitive function and reduced inflammatory markers. That’s encouraging, isn’t it?
A significant research initiative, over at Indiana University, is really digging into the link between niacin homeostasis and the gut microbiome in AD. This has even led to a clinical trial for niacin in AD, which started in 2024. Furthermore, longitudinal studies, the kind that follow people over a long time, are using advanced multi-omics techniques. That’s a fancy way of saying they’re integrating data from the gut microbiome, metabolomics (studying metabolites), and neuroimaging, all to get a comprehensive look at changes in short-chain fatty acids (SCFAs) and the overall gut microbiome composition across various stages of AD. The ultimate goal, of course, is to build robust diagnostic models for preclinical AD, before symptoms even show up.
But, and there’s always a “but,” several key challenges persist in this field. A big hurdle is definitively proving a causal relationship between gut dysbiosis and neural dysfunction in AD. While we’ve seen strong associations, it’s still complex to figure out whether changes in the gut microbiota are a direct cause or just a consequence of AD pathology. That whole “chicken or egg” dilemma, you know? But the scientific community is tackling this head-on, with more and more longitudinal, multi-omics studies. They’re moving beyond just static observations to dynamic, temporal analyses, which are crucial for inferring causal links. It shows the field is really maturing, actively taking on its core challenges.
Another challenge? The sheer complexity and huge individual variability of the gut microbiome. It’s a massive hurdle for developing standardized, universally effective interventions. And inconsistent findings across studies? They often come down to differences in how the research is done: variations in sample size, experimental methods, participant age, and even a lack of detailed data, like a comprehensive history of antibiotic use or specific types of fiber consumed. Standardizing protocols for microbiome analysis and validating these methods, well, those are major hurdles that absolutely need to be cleared.
What’s more, many current studies looking at microbiome modulation are relatively short-term. To truly understand the sustained effects of these interventions and their long-term impact on AD progression, we desperately need rigorous and extended longitudinal studies. And finally, data from certain studies, like Genome-Wide Association Studies (GWAS), often include participants primarily from specific ancestries, which can limit how broadly we can apply those findings to the general population. It’s something to keep in mind.
Looking ahead, though, the field is absolutely poised for significant advancements. The future of microbiome-based therapies for AD? It’s expected to involve highly personalized approaches. This means developing anti-inflammatory diets designed using systems biology, and multi-functional drug designs that target multiple areas of the central nervous system, aiming for both symptom relief and neuroprotective properties. Integrating diverse biological markers, including microbiome signatures, genetic profiles, proteomic data, and advanced neuroimaging, will be crucial for improving diagnostic accuracy and making AD monitoring more efficient.
Research is also moving towards developing new techniques for more selective gut microbiota modulation. This includes innovative strategies like microbial encapsulation, the therapeutic use of bacteriophages, employing microbial enzyme modulators, and even engineering microbes to produce specific beneficial metabolites. All of this, of course, to allow for more controlled and efficient interventions. And there’s a critical need for more robust, well-designed human clinical trials to truly translate those promising preclinical findings into validated clinical applications. Further in-depth research into how environment-based factors like physical exercise and stress impact the gut microbiome is also warranted, as these represent novel and accessible therapeutic strategies for AD.
Despite the inherent complexity and individual variability of the gut microbiome, its relative accessibility compared to the brain really positions it as a highly promising and practical “clinical window” for both early detection and therapeutic intervention in AD. Direct brain interventions and early AD diagnosis through brain imaging or biopsies? They’re often invasive and costly, limiting widespread use. CSF biomarkers, while valuable, are also invasive. But the gut? It’s highly accessible for non-invasive sampling, think fecal samples, and modification, like diet or oral probiotics. While the gut microbiome is indeed complex and variable, the potential to leverage “specific microbial signatures” and “detectable early microbiome changes to predict the onset or progression” for diagnostic purposes, coupled with the ease of administering gut-targeted therapies, makes it a uniquely pragmatic target. This implies that despite the scientific complexities, the practical accessibility of the gut makes it an exceptionally attractive and viable avenue for future AD management, potentially revolutionizing early intervention and treatment strategies by focusing on a more approachable system. It’s a truly exciting time for this research.
Conclusion: A New Dawn for Alzheimer’s Research?
The scientific evidence is just piling up, overwhelmingly supporting this profound and multifaceted connection between our gut microbiome and the very development of Alzheimer’s disease. The gut-brain axis is clearly functioning as a critical, two-way communication network. And when that delicate balance in the gut microbiota gets thrown off, what we call dysbiosis, it can actually kick off and then worsen AD pathology through a whole diverse array of mechanisms. We’re talking about the production and movement of neurotoxins like BF-LPS, the triggering of systemic and neuroinflammation, the weakening of crucial neural barriers like the gut lining and the blood-brain barrier, and even the modulation of amyloid-beta and tau protein aggregation. The understanding that this AD-linked pathway can originate in the gut and stay active throughout our lives, well, it fundamentally reframes how we think about AD etiology, shifting our focus squarely towards lifelong prevention and early intervention. It’s a game-changer, really.
This rapidly evolving understanding of these intricate gut-brain mechanisms is opening up some incredibly promising avenues for developing novel diagnostic tools and, crucially, new therapeutic strategies for AD. Interventions, ranging from simple, fundamental dietary modifications, like just eating more fiber, to the targeted use of probiotics and prebiotics, and even more advanced approaches like Fecal Microbiota Transplantation (FMT) and niacin pathway modulation, all collectively represent a new and truly exciting frontier in AD prevention and treatment. These diverse, multi-modal interventions actually blur the traditional lines between prevention and treatment, suggesting a continuous spectrum of care. It’s not one or the other; it’s both.
While yes, significant challenges still persist, especially in definitively proving causality, dealing with the huge individual variability in microbiome composition, and standardizing research methods, the field is, without a doubt, advancing at a rapid pace. Ongoing longitudinal studies, coupled with sophisticated multi-omics research, are poised to bridge those critical knowledge gaps, moving us closer to a more causal understanding of the gut’s role. The future of Alzheimer’s disease management it’s increasingly likely to involve personalized, gut-targeted approaches. These will leverage the accessibility and modifiability of the microbiome for earlier detection, more effective prevention, and sustained therapeutic benefits, ultimately transforming how this devastating neurodegenerative disorder is understood and, hopefully, finally combated. It’s a hopeful thought, isn’t it?